Entitlement Eligibility Guideline (EEG)
Date created: 31 March 2025
ICD-11 code: MG30.01
VAC medical code: 72910 Fibromyalgia syndrome
This publication is available upon request in alternate formats.
Full document – PDF Version
Definition
Fibromyalgia, also known as fibromyalgia syndrome, is one of the leading causes of chronic widespread pain (CWP). While pain is its primary and distinguishing characteristic, fibromyalgia is a complex set of symptoms that also include fatigue, sleep disturbances, and functional symptoms (medical symptoms not explained by structural or pathological causes) that may significantly impact the quality of life and functional ability of individuals.
For the purposes of this entitlement eligibility guideline (EEG), the following conditions are included:
- fibromyalgia
- fibromyalgia syndrome.
Diagnostic standard
Diagnosis by a qualified physician (rheumatologist, family physician), nurse practitioner, or physician assistant (within their scope of practice) is required.
The diagnosis of fibromyalgia is primarily clinically based. The ACTTION-APS Pain Taxonomy (AAPT) diagnostic criteria is a clinically useful diagnostic system and consistent across chronic pain disorders.
AAPT diagnostic criteria for fibromyalgia are as follows:
- Multisite pain (MSP): Defined as pain in six or more out of nine possible body sites (Figure 1: Pain sites of the body).
- Sleep problems or fatigue: The presence of moderate to severe sleep problems or fatigue is required. These symptoms must be judged to be of at least moderate severity by the healthcare professional.
- Duration of symptoms: MSP, along with fatigue or sleep problems, must be present for at least 3 months.
- Other disorders: The presence of another pain disorder or related symptoms does not rule out a diagnosis of fibromyalgia. However, a clinical assessment is recommended to evaluate any condition that could fully explain the patient’s symptoms or contribute to the severity of the symptoms.
Figure 1: Pain sites of the body
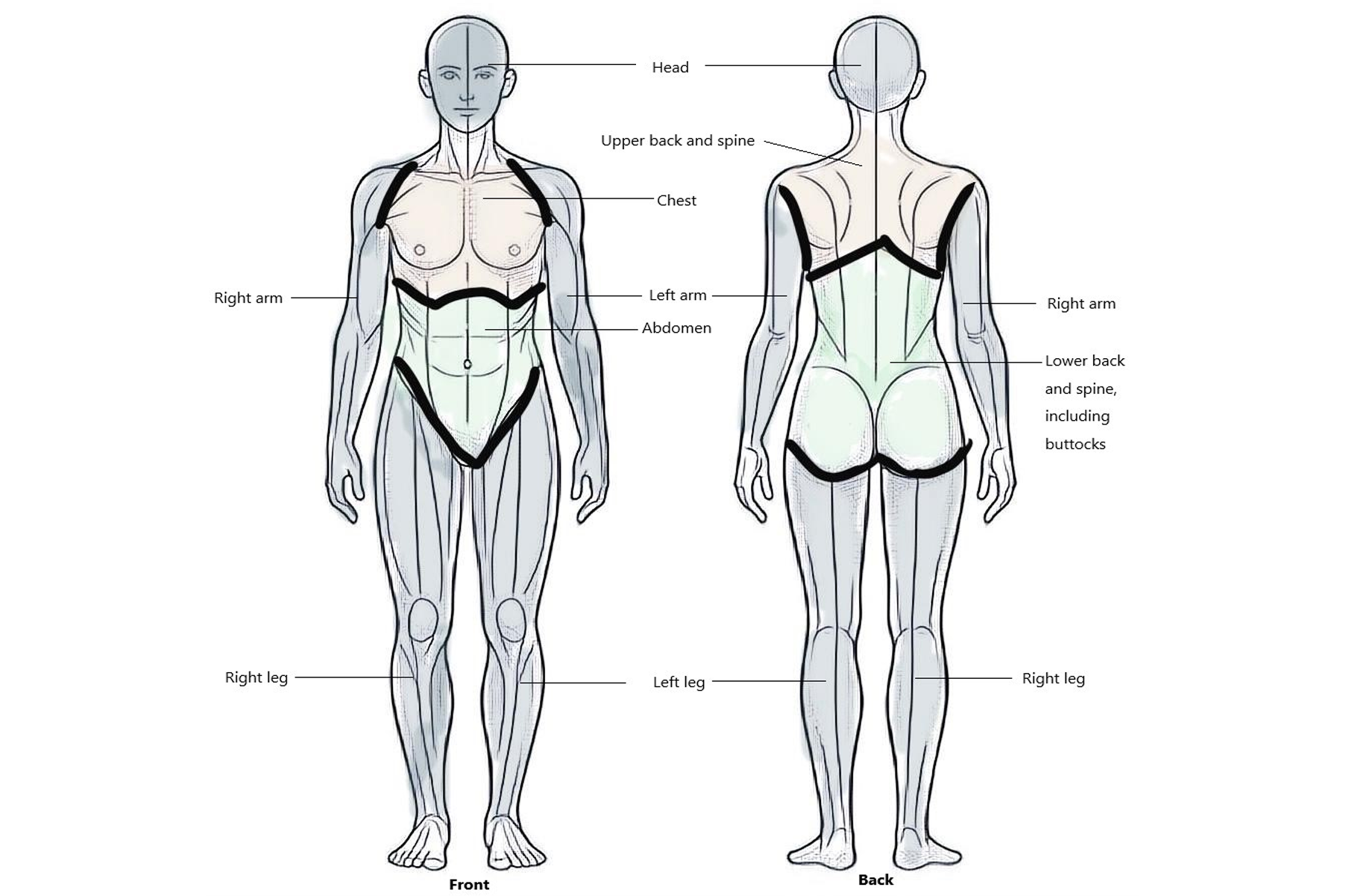
Fibromyalgia is identified by the presence of pain in six or more of nine commonly affected areas of the body. These nine pain sites include the head (both front and back), upper back, spine, chest, arms (left and right), abdomen, lower back, spine including the buttocks, and legs (left and right). Source: Veterans Affairs Canada (2024).
Anatomy and physiology
This section represents the current understanding of the anatomy and pathophysiology of fibromyalgia. It is based on the current scientific and medical literature available at the time of publication of this EEG. Review of this evidence supports that fibromyalgia results from a combination of factors involving genetic predisposition, individual experiences, emotional cognitive factors, and the mind-body relationship.
Fibromyalgia is characterized by widespread pain, sleep disturbances, mood disorders, and other symptoms that result from dysregulation of the nociceptive part of the peripheral and central nervous system. This dysregulation leads to nociplastic pain, where pain arises from altered processing of pain signals without clear evidence of tissue damage.
Overall, the pathophysiology of fibromyalgia is multifactorial, involving a complex interplay between the central and peripheral nervous systems, immune response, hormonal regulation, and genetic susceptibility. Genetic predisposition combined with environmental triggers (such as physical or emotional trauma) appear to play a role in the syndrome's onset.
Although the details of the mechanisms of dysregulation are still under investigation, the following physiological processes and changes have been substantiated in the current medical and scientific literature.
Central nervous system sensitization: Fibromyalgia is associated with alterations in the central nervous system that lead to increased sensitivity to pain. This central sensitization involves enhanced processing and decreased inhibition of pain signals in the brain and spinal cord, contributing to widespread pain.
Neurotransmitter dysregulation: Neurotransmitter levels in fibromyalgia (including serotonin, norepinephrine, and dopamine) may be poorly regulated and affect pain perception, mood regulation, and sleep.
Immune system involvement: There are abnormal levels of proinflammatory chemical mediators in the blood and cerebrospinal fluid of individuals with fibromyalgia, suggesting a low-grade inflammatory response.
Autonomic nervous system dysfunction: The autonomic nervous system may be impaired, affecting bodily functions such as temperature regulation issues and genitourinary and gastrointestinal symptoms.
Hormonal imbalances: Abnormal stress hormone levels, including cortisol, have been observed in fibromyalgia patients, indicating a potential link between stress response systems and fibromyalgia symptoms.
Oxidative stress and mitochondrial dysfunction: Oxidative stress and mitochondrial dysfunction in fibromyalgia may contribute to muscle pain and fatigue. Abnormalities in mitochondrial function may lead to energy deficits and influence the production of pain related neurotransmitters.
Clinical features
The clinical presentation of fibromyalgia is complex and involves a constellation of symptoms beyond the feature of chronic widespread pain. Patients with fibromyalgia often experience deep, aching pains that can vary in intensity and location, affecting muscles, joints, and soft tissues. The pain is typically persistent, with fluctuations in severity, and can be aggravated by environmental and psychological stressors.
Widespread pain: The primary symptom is a persistent ache or sharp pain across various parts of the body and is often described as coming from the muscles or joints. This pain is widespread and not confined to one specific area, typically presenting at bodily sites depicted in Figure 1: Pain sites of the body.
Fatigue: Individuals with fibromyalgia report a profound sense of exhaustion that is not relieved by rest or sleep, affecting daily activities and quality of life. This fatigue can be physical and mental, leading to cognitive difficulties known as "fibro fog," which includes problems with concentration, memory, and decision making.
Sleep disturbances: There is often report of experiencing nonrestorative sleep, difficulty falling asleep, or staying asleep, contributing to daytime fatigue and cognitive impairments.
Mood disorders: In individuals with fibromyalgia there is a high prevalence of mood disturbances, including depression and anxiety, which can exacerbate the perception of pain and further impair function.
Autonomic symptoms: Symptoms indicative of autonomic nervous system dysfunction, such as gastrointestinal symptoms, headaches, genitourinary symptoms, and temperature sensitivity are frequently reported.
Sensory overload: People with fibromyalgia may experience heightened sensitivity to stimuli, including light, noise, and temperature changes, often referred to as sensory overload or hypersensitivity.
The evidence suggests a significant association between exposure to stressors and adult fibromyalgia, with the strongest associations observed for physical abuse. The relationship between stressful life events, including both physical injuries and psychological stress such as posttraumatic stress disorder (PTSD), and the development of fibromyalgia highlights the complex interaction between environmental stressors and biological vulnerabilities.
Fibromyalgia demonstrates a higher prevalence in females compared to males, with an estimated 3.4% adult females and 0.5% of adult males affected. The onset of fibromyalgia symptoms can occur at any age but is most diagnosed in middle adulthood.
Entitlement considerations
In this section
Section A: Causes and/or aggravation
Section B: Medical conditions which are to be included in entitlement/assessment
Section A: Causes and/or aggravation
For Veterans Affairs Canada (VAC) entitlement purposes, the following factors are accepted to cause or aggravate the conditions included in the Definition section of this EEG, and may be considered along with the evidence to assist in establishing a relationship to service. The factors have been determined based on a review of up-to-date scientific and medical literature, as well as evidence-based medical best practices. Factors other than those listed may be considered, however consultation with a disability consultant or medical advisor is recommended.
The timelines cited below are for guidance purposes. Each case should be adjudicated on the evidence provided and its own merits.
Factors
- Having experienced a significant physical trauma. Significant trauma should have ongoing symptoms that require medical attention. The clinical onset or aggravation of fibromyalgia should occur within one year of the traumatic event. Examples may include, but are not limited to, cervical and lumbar spine injuries.
- Having a clinically significant psychiatric condition within two years prior to the clinical onset or aggravation of fibromyalgia.
Note: For VAC purposes, clinically significant means requiring ongoing treatment and clinical management.
- Directly experiencing a traumatic event(s) within one year before clinical onset or aggravation of fibromyalgia.
Traumatic events include, but are not limited to:
- emotional trauma
- exposure to military combat
- threatened or actual physical assault
- threatened or actual sexual trauma
- being kidnapped
- being taken hostage
- being in a terrorist attack
- being tortured
- incarceration as a prisoner of war
- being in a natural or human-made disaster
- being in a severe motor vehicle accident
- killing or injuring a person
- experiencing a sudden, catastrophic medical incident
- experiencing an acute, severe, emotional stressor
- medical trauma
- moral injury
- sexual harassment
- repeated exposure to prejudicial or unjust treatment.
- Having an autoimmune inflammatory disease at the time of clinical onset or aggravation of fibromyalgia. Some examples of autoimmune inflammatory disease include, but are not limited to:
- rheumatoid arthritis
- ankylosing spondylitis
- systemic lupus erythematosus.
- Inability to obtain appropriate clinical management of fibromyalgia.
Section B: Medical conditions which are to be included in entitlement/assessment
Section B provides a list of diagnosed medical conditions which are considered for VAC purposes to be included in the entitlement and assessment of fibromyalgia.
- Diffuse myofascial pain syndrome
- Chronic widespread pain (CWP)
- Myalgic encephalomyelitis (ME)
- Chronic fatigue syndrome (CFS)
- Myofascial pain syndrome
Note: Physical or psychological manifestations such as, but not limited to, changes in mood, anxiety, sleep disturbance, fatigue, headaches, temperature dysregulation, sensory changes, gastrointestinal and/or genitourinary symptoms and soft tissue/myofascial pain are included in the entitlement and assessment of fibromyalgia. Where symptoms develop into a separate diagnosed disorder, consequential entitlement may be considered.
Section C: Common medical conditions which may result, in whole or in part, from fibromyalgia and/or its treatment
No consequential medical conditions were identified at the time of the publication of this EEG. If the merits of the case and medical evidence indicate that a possible consequential relationship may exist, consultation with a disability consultant or medical advisor is recommended.
Links
Related VAC guidance and policy:
- Adjustment Disorder – Entitlement Eligibility Guidelines
- Ankylosing Spondylitis – Entitlement Eligibility Guidelines
- Anxiety Disorders – Entitlement Eligibility Guidelines
- Bipolar and Related Disorders - Entitlement Eligibility Guidelines
- Depressive Disorders - Entitlement Eligibility Guidelines
- Feeding and Eating Disorders - Entitlement Eligibility Guidelines
- Posttraumatic Stress Disorder - Entitlement Eligibility Guidelines
- Rheumatoid Arthritis – Entitlement Eligibility Guidelines
- Schizophrenia - Entitlement Eligibility Guidelines
- Substance Use Disorders - Entitlement Eligibility Guidelines
- Pain and Suffering Compensation – Policies
- Royal Canadian Mounted Police Disability Pension Claims – Policies
- Dual Entitlement – Disability Benefits – Policies
- Establishing the Existence of a Disability – Policies
- Disability Benefits in Respect of Peacetime Military Service – The Compensation Principle – Policies
- Disability Benefits in Respect of Wartime and Special Duty Service – The Insurance Principle – Policies
- Disability Resulting from a Non-Service Related Injury or Disease – Policies
- Consequential Disability – Policies
- Benefit of Doubt – Policies
References as of 31 March 2025
Ablin, J. N., Cohen, H., Neumann, L., Kaplan, Z., & Buskila, D. (2008). Coping styles in fibromyalgia: Effect of co-morbid posttraumatic stress disorder. Rheumatology International, 28(7), Article 7. https://doi.org/10.1007/s00296-007-0496-1
Adamowicz, J. L., Thomas, E. B. K., Lund, B. C., Driscoll, M. A., Weg, M. V., & Hadlandsmyth, K. (2023). A population-based investigation into the prevalence of chronic fatigue syndrome in United States military Veterans with chronic pain. Fatigue: Biomedicine, Health & Behavior, 11(2–4), Article 2–4. https://doi.org/10.1080/21641846.2023.2239977
Ahmad, J., & Tagoe, C. E. (2014). Fibromyalgia and chronic widespread pain in autoimmune thyroid disease. Clinical Rheumatology, 33(7), Article 7. https://doi.org/10.1007/s10067-014-2490-9
Ahmed, S., & Lawrence, A. (2020). PATHOGENESIS OF FIBROMYALGIA IN PATIENTS WITH AUTOIMMUNE DISEASES: SCOPING REVIEW FOR HYPOTHESIS GENERATION. Central Asian Journal of Medical Hypotheses and Ethics, 1(1), Article 1. https://doi.org/10.47316/cajmhe.2020.1.1.06
Akkus, S., Delibas, N., & Tamer, M. N. (2000). Do sex hormones play a role in fibromyalgia? Rheumatology, 39(10), Article 10. https://doi.org/10.1093/rheumatology/39.10.1161
Alciati, A., Atzeni, F., Caldirola, D., Perna, G., & Sarzi-Puttini, P. (2020). The Co-Morbidity between Bipolar and Panic Disorder in Fibromyalgia Syndrome. Journal of Clinical Medicine, 9(11), Article 11. https://doi.org/10.3390/jcm9113619
Arnold, L. M., Bennett, R. M., Crofford, L. J., Dean, L. E., Clauw, D. J., Goldenberg, D. L., Fitzcharles, M.-A., Paiva, E. S., Staud, R., Sarzi-Puttini, P., Buskila, D., & Macfarlane, G. J. (2019). AAPT Diagnostic Criteria for Fibromyalgia. The Journal of Pain, 20(6), 611–628. https://doi.org/10.1016/j.jpain.2018.10.008
Arnold, L. M., Hudson, J. I., Keck, P. E., Auchenbach, M. B., Javaras, K. N., & Hess, E. V. (2006). Comorbidity of Fibromyalgia and Psychiatric Disorders. The Journal of Clinical Psychiatry, 67(08), Article 08. https://doi.org/10.4088/JCP.v67n0807
Arout, C. A., Sofuoglu, M., Bastian, L. A., & Rosenheck, R. A. (2018). Gender Differences in the Prevalence of Fibromyalgia and in Concomitant Medical and Psychiatric Disorders: A National Veterans Health Administration Study. Journal of Women’s Health (2002), 27(8), Article 8. https://doi.org/10.1089/jwh.2017.6622
Australian Government Repatriation Medical Authority. (2021) Statement of Principles concerning fibromyalgia (Balance of Probabilities) (No. 108 of 2021). SOPs - Repatriation Medical Authority
Australian Government Repatriation Medical Authority. (2021) Statement of Principles concerning fibromyalgia (Reasonable Hypothesis) (No. 107 of 2021). SOPs - Repatriation Medical Authority
Bennett, R. (1998). Fibromyalgia, chronic fatigue syndrome, and myofascial pain: Current Opinion in Rheumatology, 10(2), Article 2. https://doi.org/10.1097/00002281-199803000-00002
Bernik, M., Sampaio, T. P. A., & Gandarela, L. (2013). Fibromyalgia Comorbid with Anxiety Disorders and Depression: Combined Medical and Psychological Treatment. Current Pain and Headache Reports, 17(9), Article 9. https://doi.org/10.1007/s11916-013-0358-3
Bilge, U., Sari, Y. E., Balcioglu, H., Yasar Bilge, N. S., Kasifoglu, T., Kayhan, M., & Unluoglu, I. (2018). Prevalence of comorbid diseases in patients with fibromyalgia: A retrospective cross-sectional study. JPMA. The Journal of the Pakistan Medical Association, 68(5), Article 5.
Buhler, J. N. (2022). Biopsychosocial Determinants of Chronic Pain Amongst Canadian Armed Forces Veterans.
Buskila, D. (2000). Fibromyalgia, chronic fatigue syndrome, and myofascial pain syndrome. Current Opinion in Rheumatology, 12(2), Article 2. https://doi.org/10.1097/00002281-200003000-00005
Buskila, D., & Cohen, H. (2007). Comorbidity of fibromyalgia and psychiatric disorders. Current Pain and Headache Reports, 11(5), Article 5. https://doi.org/10.1007/s11916-007-0214-4
Buskila, D., Neumann, L., Alhoashle, A., & Abu-Shakra, M. (2000). Fibromyalgia syndrome in men. Seminars in Arthritis and Rheumatism, 30(1), Article 1. https://doi.org/10.1053/sarh.2000.8363
Cakit, B. D., Taskin, S., Nacir, B., Unlu, I., Genc, H., & Erdem, H. R. (2010). Comorbidity of fibromyalgia and cervical myofascial pain syndrome. Clinical Rheumatology, 29(4), Article 4. https://doi.org/10.1007/s10067-009-1342-5
Cao, B., Xu, Q., Shi, Y., Zhao, R., Li, H., Zheng, J., Liu, F., Wan, Y., & Wei, B. (2024). Pathology of pain and its implications for therapeutic interventions. Signal Transduction and Targeted Therapy, 9(1), 155. https://doi.org/10.1038/s41392-024-01845-w
Carta, M. G., Moro, M. F., Pinna, F. L., Testa, G., Cacace, E., Ruggiero, V., Piras, M., Romano, F., Minerba, L., Machado, S., Freire, R. C., Nardi, A. E., & Sancassiani, F. (2018). The impact of fibromyalgia syndrome and the role of comorbidity with mood and post-traumatic stress disorder in worsening the quality of life. International Journal of Social Psychiatry, 64(7), Article 7. https://doi.org/10.1177/0020764018795211
Chang, M.-H., Hsu, J.-W., Huang, K.-L., Su, T.-P., Bai, Y.-M., Li, C.-T., Yang, A. C., Chang, W.-H., Chen, T.-J., Tsai, S.-J., & Chen, M.-H. (2015). Bidirectional Association Between Depression and Fibromyalgia Syndrome: A Nationwide Longitudinal Study. The Journal of Pain, 16(9), Article 9. https://doi.org/10.1016/j.jpain.2015.06.004
Clauw, D. J. (2015). Fibromyalgia and Related Conditions. Mayo Clinic Proceedings, 90(5), Article 5. https://doi.org/10.1016/j.mayocp.2015.03.014
Conversano, C., Ciacchini, R., Orrù, G., Bazzichi, M. L., Gemignani, A., & Miniati, M. (2021). Gender differences on psychological factors in fibromyalgia: A systematic review on the male experience. Clinical and Experimental Rheumatology, 39(3), Article 3. https://doi.org/10.55563/clinexprheumatol/73g6np
Coskun Benlidayi, I. (2019). Role of inflammation in the pathogenesis and treatment of fibromyalgia. Rheumatology International, 39(5), 781–791. https://doi.org/10.1007/s00296-019-04251-6
Creed, F. (2020). A review of the incidence and risk factors for fibromyalgia and chronic widespread pain in population-based studies. Pain, 161(6), Article 6. https://doi.org/10.1097/j.pain.0000000000001819
D’Aoust, R. F., Rossiter, A. G., Elliott, A., Ji, M., Lengacher, C., & Groer, M. (2017). Women Veterans, a Population at Risk for Fibromyalgia: The Associations Between Fibromyalgia, Symptoms, and Quality of Life. Military Medicine, 182(7), Article 7. https://doi.org/10.7205/MILMED-D-15-00557
Das, D., & Choy, E. (2023). Non-inflammatory pain in inflammatory arthritis. Rheumatology, 62(7), 2360–2365. https://doi.org/10.1093/rheumatology/keac671
Di Tommaso Morrison, M. C., Carinci, F., Lessiani, G., Spinas, E., Kritas, S. K., Ronconi, G., Caraffa, A., & Conti, P. (2017). Fibromyalgia and bipolar disorder: Extent of comorbidity and therapeutic implications. Journal of Biological Regulators and Homeostatic Agents, 31(1), Article 1.
Fernández-de-las-Peñas, C., & Arendt-Nielsen, L. (2016). Myofascial Pain and Fibromyalgia: Two Different but Overlapping Disorders. Pain Management, 6(4), Article 4. https://doi.org/10.2217/pmt-2016-0013
Fitzcharles, M. ‐A., Perrot, S., & Häuser, W. (2018). Comorbid fibromyalgia: A qualitative review of prevalence and importance. European Journal of Pain, 22(9), Article 9. https://doi.org/10.1002/ejp.1252
Fitzcharles, M.-A., Cohen, S. P., Clauw, D. J., Littlejohn, G., Usui, C., & Häuser, W. (2021). Nociplastic pain: Towards an understanding of prevalent pain conditions. The Lancet, 397(10289), 2098–2110. https://doi.org/10.1016/S0140-6736(21)00392-5
Galvez-Sánchez, C. M., & Reyes del Paso, G. A. (2020). Diagnostic Criteria for Fibromyalgia: Critical Review and Future Perspectives. Journal of Clinical Medicine, 9(4), 1219. https://doi.org/10.3390/jcm9041219
Gardoki-Souto, I., Redolar-Ripoll, D., Fontana, M., Hogg, B., Castro, M. J., Blanch, J. M., Ojeda, F., Solanes, A., Radua, J., Valiente-Gómez, A., Cirici, R., Pérez, V., Amann, B. L., & Moreno-Alcázar, A. (2022). Prevalence and Characterization of Psychological Trauma in Patients with Fibromyalgia: A Cross-Sectional Study. Pain Research and Management, 2022, 1–16. https://doi.org/10.1155/2022/2114451
Gau, S.-Y., Leong, P.-Y., Lin, C.-L., Tsou, H.-K., & Wei, J. C.-C. (2021). Higher Risk for Sjögren’s Syndrome in Patients With Fibromyalgia: A Nationwide Population-Based Cohort Study. Frontiers in Immunology, 12, 640618. https://doi.org/10.3389/fimmu.2021.640618
Gerber, M. R., Bogdan, K. M., Haskell, S. G., & Scioli, E. R. (2018). Experience of Childhood Abuse and Military Sexual Trauma Among Women Veterans with Fibromyalgia. Journal of General Internal Medicine, 33(12), 2030–2031. https://doi.org/10.1007/s11606-018-4594-4
Gerwin, R. D. (2005). A Review of Myofascial Pain and Fibromyalgia – Factors that Promote Their Persistence. Acupuncture in Medicine, 23(3), Article 3. https://doi.org/10.1136/aim.23.3.121
Giacomelli, C., Talarico, R., Bombardieri, S., & Bazzichi, L. (2013). The interaction between autoimmune diseases and fibromyalgia: Risk, disease course and management. Expert Review of Clinical Immunology, 9(11), Article 11. https://doi.org/10.1586/1744666X.2013.849440
Gilheaney, Ó., & Chadwick, A. (2024). The Prevalence and Nature of Eating and Swallowing Problems in Adults with Fibromyalgia: A Systematic Review. Dysphagia, 39(1), 92–108. https://doi.org/10.1007/s00455-023-10597-8
Greenbaum, H., Weil, C., Chodick, G., Shalev, V., & Eisenberg, V. H. (2019). Evidence for an association between endometriosis, fibromyalgia, and autoimmune diseases. American Journal of Reproductive Immunology, 81(4), Article 4. https://doi.org/10.1111/aji.13095
Haliloglu, S., Ekinci, B., Uzkeser, H., Sevimli, H., Carlioglu, A., & Macit, P. M. (2017). Fibromyalgia in patients with thyroid autoimmunity: Prevalence and relationship with disease activity. Clinical Rheumatology, 36(7), Article 7. https://doi.org/10.1007/s10067-017-3556-2
Häuser, W., Galek, A., Erbslöh-Möller, B., Köllner, V., Kühn-Becker, H., Langhorst, J., Petermann, F., Prothmann, U., Winkelmann, A., Schmutzer, G., Brähler, E., & Glaesmer, H. (2013). Posttraumatic stress disorder in fibromyalgia syndrome: Prevalence, temporal relationship between posttraumatic stress and fibromyalgia symptoms, and impact on clinical outcome. Pain, 154(8), Article 8. https://doi.org/10.1016/j.pain.2013.03.034
Jeffery, D. D., Bulathsinhala, L., Kroc, M., & Dorris, J. (2014). Prevalence, Health Care Utilization, and Costs of Fibromyalgia, Irritable Bowel, and Chronic Fatigue Syndromes in the Military Health System, 2006–2010. Military Medicine, 179(9), Article 9. https://doi.org/10.7205/MILMED-D-13-00419
Kaleycheva, N., Cullen, A. E., Evans, R., Harris, T., Nicholson, T., & Chalder, T. (2021). The role of lifetime stressors in adult fibromyalgia: Systematic review and meta-analysis of case-control studies. Psychological Medicine, 51(2), 177–193. https://doi.org/10.1017/S0033291720004547
Kaplan, C. M., Kelleher, E., Irani, A., Schrepf, A., Clauw, D. J., & Harte, S. E. (2024). Deciphering nociplastic pain: Clinical features, risk factors and potential mechanisms. Nature Reviews Neurology, 20(6), 347–363. https://doi.org/10.1038/s41582-024-00966-8
Katz, J. D., Mamyrova, G., Guzhva, O., & Furmark, L. (2010). Gender bias in diagnosing fibromyalgia. Gender Medicine, 7(1), Article 1. https://doi.org/10.1016/j.genm.2010.01.003
Kleykamp, B. A., Ferguson, M. C., McNicol, E., Bixho, I., Arnold, L. M., Edwards, R. R., Fillingim, R., Grol-Prokopczyk, H., Turk, D. C., & Dworkin, R. H. (2021). The Prevalence of Psychiatric and Chronic Pain Comorbidities in Fibromyalgia: An ACTTION systematic review. Seminars in Arthritis and Rheumatism, 51(1), Article 1. https://doi.org/10.1016/j.semarthrit.2020.10.006
Kosek, E., Clauw, D., Nijs, J., Baron, R., Gilron, I., Harris, R. E., Mico, J.-A., Rice, A. S. C., & Sterling, M. (2021). Chronic nociplastic pain affecting the musculoskeletal system: Clinical criteria and grading system. Pain, 162(11), 2629–2634. https://doi.org/10.1097/j.pain.0000000000002324
Kudlow, P. A., Rosenblat, J. D., Weissman, C. R., Cha, D. S., Kakar, R., McIntyre, R. S., & Sharma, V. (2015). Prevalence of fibromyalgia and co-morbid bipolar disorder: A systematic review and meta-analysis. Journal of Affective Disorders, 188, 134–142. https://doi.org/10.1016/j.jad.2015.08.030
Lawrence‐Wolff, K. M., Higgs, J. B., Young‐McCaughan, S., Mintz, J., Foa, E. B., Resick, P. A., Kelly, K. M., Maurer, D. M., Borah, A. M., Yarvis, J. S., Litz, B. T., Hildebrand, B. A., Williamson, D. E., Peterson, A. L., & for the STRONG STAR Consortium. (2023). Prevalence of Fibromyalgia Syndrome in Military Personnel. Arthritis Care & Research, 75(3), Article 3. https://doi.org/10.1002/acr.24801
Loganathan, M., Ladani, A., & Lippmann, S. (2020). Fibromyalgia, Sjogren’s & depression: Linked? Postgraduate Medicine, 132(7), Article 7. https://doi.org/10.1080/00325481.2020.1758426
Ma, K. S., Lai, J., Veeravalli, J. J., Chiu, L., Van Dyke, T. E., & Wei, J. C. (2022). Fibromyalgia and periodontitis: Bidirectional associations in population‐based 15‐year retrospective cohorts. Journal of Periodontology, 93(6), Article 6. https://doi.org/10.1002/JPER.21-0256
Marshall, A., Rapteas, L., Burgess, J., Riley, D., Anson, M., Matsumoto, K., Bennett, A., Kaye, S., Marshall, A., Dunham, J., Fallon, N., Zhao, S. S., Pritchard, A., Goodson, N., Malik, R. A., Goebel, A., Frank, B., & Alam, U. (2024). Small fibre pathology, small fibre symptoms and pain in fibromyalgia syndrome. Scientific Reports, 14(1), 3947. https://doi.org/10.1038/s41598-024-54365-6
Martínez-Jauand, M., Sitges, C., Femenia, J., Cifre, I., González, S., Chialvo, D., & Montoya, P. (2013). Age-of-onset of menopause is associated with enhanced painful and non-painful sensitivity in fibromyalgia. Clinical Rheumatology, 32(7), Article 7. https://doi.org/10.1007/s10067-013-2212-8
Maunder, L., Marriott, E., Katz, J., & Salomons, T. V. (2022). Mechanisms of heightened pain-related disability in Canadian Armed Forces members and Veterans with comorbid chronic pain and PTSD. Journal of Military, Veteran and Family Health, 8(3), Article 3. https://doi.org/10.3138/jmvfh-2022-0011
May, K. P., West, S. G., Baker, M. R., & Everett, D. W. (1993). Sleep apnea in male patients with the fibromyalgia syndrome. The American Journal of Medicine, 94(5), Article 5. https://doi.org/10.1016/0002-9343(93)90085-4
Mohanty, A. F., Helmer, D. A., Muthukutty, A., McAndrew, L. M., Carter, M. E., Judd, J., Garvin, J. H., RHIA, CPHQ, CCS, FAHIMA, Samore, M. H., & Gundlapalli, A. V. (2016). Fibromyalgia syndrome care of Iraq- and Afghanistan-deployed Veterans in Veterans Health Administration. Journal of Rehabilitation Research and Development, 53(1), Article 1. https://doi.org/10.1682/JRRD.2014.10.0265
Mork, P. J., & Nilsen, T. I. L. (2012). Sleep problems and risk of fibromyalgia: Longitudinal data on an adult female population in Norway. Arthritis & Rheumatism, 64(1), Article 1. https://doi.org/10.1002/art.33346
Moroni, L., Bianchi, I., & Lleo, A. (2012). Geoepidemiology, gender and autoimmune disease. Autoimmunity Reviews, 11(6–7), Article 6–7. https://doi.org/10.1016/j.autrev.2011.11.012
Morrison, M. C., Carinci, F., Lessiani, G., Spinas, E., Kritas, S., Ronconi, G., Caraffa, A., & Conti, P. (2017). Fibromyalgia and bipolar disorder: Extent of comorbidity and therapeutic implications. Journal of Biological Regulators and Homeostatic Agents, 31, 17–20.
Murphy, A. E., Minhas, D., Clauw, D. J., & Lee, Y. C. (2023). Identifying and Managing Nociplastic Pain in Individuals With Rheumatic Diseases: A Narrative Review. Arthritis Care & Research, 75(10), 2215–2222. https://doi.org/10.1002/acr.25104
Nussinovitch, U., & Shoenfeld, Y. (2012). The role of gender and organ specific autoimmunity. Autoimmunity Reviews, 11(6–7), Article 6–7. https://doi.org/10.1016/j.autrev.2011.11.001
Oran, Ö., Dönmez, A., Erdoğan, N., & Turan, M. (2002). Psychiatric Co-Morbidity Affects the Symptoms of Fibromyalgia. Physikalische Medizin, Rehabilitationsmedizin, Kurortmedizin, 12(5), Article 5. https://doi.org/10.1055/s-2002-35160
Overstreet, D. S., Strath, L. J., Jordan, M., Jordan, I. A., Hobson, J. M., Owens, M. A., Williams, A. C., Edwards, R. R., & Meints, S. M. (2023). A Brief Overview: Sex Differences in Prevalent Chronic Musculoskeletal Conditions. International Journal of Environmental Research and Public Health, 20(5), 4521. https://doi.org/10.3390/ijerph20054521
Park, S., Kwon, J.-S., Park, Y.-B., & Park, J. W. (2021). Is thyroid autoimmunity a predisposing factor for fibromyalgia? A systematic review and meta-analysis. Clinical and Experimental Rheumatology. https://doi.org/10.55563/clinexprheumatol/y3gfva
Pinto, A. M., Luís, M., Geenen, R., Palavra, F., Lumley, M. A., Ablin, J. N., Amris, K., Branco, J., Buskila, D., Castelhano, J., Castelo-Branco, M., Crofford, L. J., Fitzcharles, M.-A., Häuser, W., Kosek, E., Mease, P. J., Marques, T. R., Jacobs, J. W. G., Castilho, P., & da Silva, J. A. P. (2023). Neurophysiological and psychosocial mechanisms of fibromyalgia: A comprehensive review and call for an integrative model. Neuroscience & Biobehavioral Reviews, 151, 105235. https://doi.org/10.1016/j.neubiorev.2023.105235
Raphael, K. G., Janal, M. N., & Nayak, S. (2004). Comorbidity of Fibromyalgia and Posttraumatic Stress Disorder Symptoms in a Community Sample of Women. Pain Medicine, 5(1), Article 1. https://doi.org/10.1111/j.1526-4637.2004.04003.x
Sadr, S., Mobini, M., Tabarestani, M., Islami Parkoohi, P., & Elyasi, F. (2023). The frequency of psychiatric disorder co‐morbidities in patients with fibromyalgia: A cross‐sectional study in Iran. Nursing Open, 10(7), Article 7. https://doi.org/10.1002/nop2.1731
Sarzi-Puttini, P., Giorgi, V., Marotto, D., & Atzeni, F. (2020). Fibromyalgia: An update on clinical characteristics, aetiopathogenesis and treatment. Nature Reviews Rheumatology, 16(11), 645–660. https://doi.org/10.1038/s41584-020-00506-w
Smith, S. B., Maixner, D. W., Fillingim, R. B., Slade, G., Gracely, R. H., Ambrose, K., Zaykin, D. V., Hyde, C., John, S., Tan, K., Maixner, W., & Diatchenko, L. (2012). Large candidate gene association study reveals genetic risk factors and therapeutic targets for fibromyalgia. Arthritis & Rheumatism, 64(2), Article 2. https://doi.org/10.1002/art.33338
Torrente-Segarra, V., Salman-Monte, T. C., Rúa-Figueroa, Í., Pérez-Vicente, S., López-Longo, F. J., Galindo-Izquierdo, M., Calvo-Alén, J., Olivé-Marqués, A., Ibañez-Ruán, J., Horcada, L., Sánchez-Atrio, A., Montilla, C., Rodríguez-Gómez, M., Díez-Álvarez, E., Martinez-Taboada, V., Andreu, J. L., Fernández-Berrizbeitia, O., Hernández-Beriain, J. A., Gantes, M., … Study Group of Systemic Autoimmune Diseases of the SER (EAS-SER). (2016). Fibromyalgia prevalence and related factors in a large registry of patients with systemic lupus erythematosus. Clinical and Experimental Rheumatology, 34(2 Suppl 96), Article 2 Suppl 96.
Tzeng, N.-S., Chung, C.-H., Liu, F.-C., Chiu, Y.-H., Chang, H.-A., Yeh, C.-B., Huang, S.-Y., Lu, R.-B., Yeh, H.-W., Kao, Y.-C., Chiang, W.-S., Tsao, C.-H., Wu, Y.-F., Chou, Y.-C., Lin, F.-H., & Chien, W.-C. (2018). Fibromyalgia and Risk of Dementia—A Nationwide, Population-Based, Cohort Study. The American Journal of the Medical Sciences, 355(2), Article 2. https://doi.org/10.1016/j.amjms.2017.09.002
Veterans Affairs Canada (2024). Pain Sites of the Body. [digital].
Vishne, T., Fostick, L., Silberman, A., Kupchick, M., Rubinow, A., Amital, H., & Amital, D. (2008). Fibromyalgia among major depression disorder females compared to males. Rheumatology International, 28(9), Article 9. https://doi.org/10.1007/s00296-008-0533-8
Vun, E. (2018). An examination of chronic pain conditions and mental health correlates in a population-based survey of Canadian Forces personnel [Master’s thesis]. University of Manitoba.
Vun, E., Turner, S., Sareen, J., Mota, N., Afifi, T. O., & El-Gabalawy, R. (2018). Prevalence of comorbid chronic pain and mental health conditions in Canadian Armed Forces active personnel: Analysis of a cross-sectional survey. CMAJ Open, 6(4), Article 4. https://doi.org/10.9778/cmajo.20180093
Weir, P. T., Harlan, G. A., Nkoy, F. L., Jones, S. S., Hegmann, K. T., Gren, L. H., & Lyon, J. L. (2006). The Incidence of Fibromyalgia and Its Associated Comorbidities: A Population-Based Retrospective Cohort Study Based on International Classification of Diseases, 9th Revision Codes. JCR: Journal of Clinical Rheumatology, 12(3), Article 3. https://doi.org/10.1097/01.rhu.0000221817.46231.18
Whitehead, W. E., Palsson, O., & Jones, K. R. (2002). Systematic review of the comorbidity of irritable bowel syndrome with other disorders: What are the causes and implications? Gastroenterology, 122(4), Article 4. https://doi.org/10.1053/gast.2002.32392
Winslow, B. T. (2023). Fibromyalgia: Diagnosis and Management. 107(2).
Wolfe, F., Ablin, J., Guymer, E. K., Littlejohn, G. O., & Rasker, J. J. (2020). The Relation of Physical Comorbidity and Multimorbidity to Fibromyalgia, Widespread Pain, and Fibromyalgia-related Variables. The Journal of Rheumatology, 47(4), Article 4. https://doi.org/10.3899/jrheum.190149
Wolfe, F., Walitt, B., Perrot, S., Rasker, J. J., & Häuser, W. (2018). Fibromyalgia diagnosis and biased assessment: Sex, prevalence and bias. PLOS ONE, 13(9), Article 9. https://doi.org/10.1371/journal.pone.0203755
World Health Organization. (2022). ICD-11: International classification of diseases (11th revision). https://icd.who.int/
Yepez, D., Grandes, X. A., Talanki Manjunatha, R., Habib, S., & Sangaraju, S. L. (2022). Fibromyalgia and Depression: A Literature Review of Their Shared Aspects. Cureus. https://doi.org/10.7759/cureus.24909
Zetterman, T., Markkula, R., Partanen, J. V., Miettinen, T., Estlander, A.-M., & Kalso, E. (2021). Muscle activity and acute stress in fibromyalgia. BMC Musculoskeletal Disorders, 22(1), 183. https://doi.org/10.1186/s12891-021-04013-1